we are studying only about 20 amino acids, there are about six more found in the body. Many others are also known from a variety of sources. Amino acids are the building blocks used to make proteins and peptides. The different amino acids have interesting properties because they have a variety of structural parts which result in different polarities and solubility.

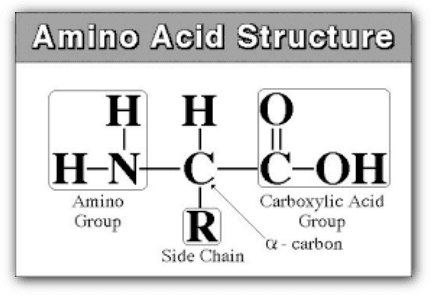
Each amino acid has at least one amine and one acid functional group as the name implies. The different properties result from variations in the structures of different R groups. The R group is often referred to as the amino acid “side chain”. Amino acids have special common names, however, a three-letter abbreviation for the name is used most of the time. Consult the amino acid table on the next page for structure, names, and abbreviations.
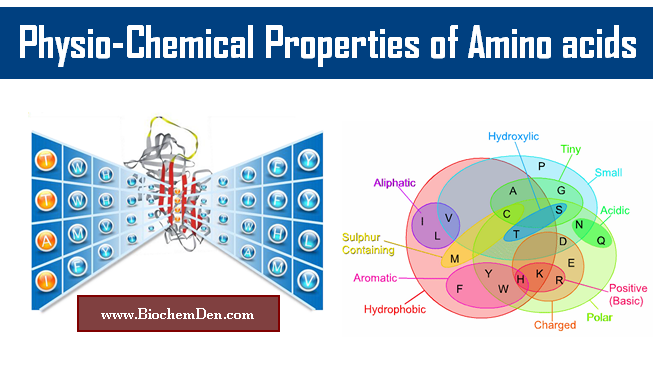
Amino acids Classification
1. Structure of Amino acids
Amino acids are a group of organic compounds containing two functional groups – amino and carboxyl. The amino group (-NH2) is basic while the carboxyl group (-COOH) is acidic in nature.
2. Classification of Amino acids
Amino acids can be classified into 4 types:
- based on the position of “-NH2”
- based on the composition of “-R” side chain
- based on the Nutritional requirement
- based on the Metabolic Fate
3. Properties of Amino acids
- Solubility: Most of the amino acids are usually soluble in water, and insoluble in organic solvents.
- Melting Point: Amino acids are generally melted at a higher temperature of ten above 2000C.
- Taste: Amino acids may be sweet (Gly, Ala & Val), tasteless (Leu) or Bitter (Arg & Ile).
- Optical Properties: All amino acids possess optical isomers due to the presence of asymmetric α-carbon atoms.
4. Zwitter ions and Isoelectric pH
Amino acid physical properties indicate a “salt-like” behavior. Amino acids are crystalline solids with relatively high melting points, and most are quite soluble in water and insoluble in non-polar solvents. In solution, the amino acid molecule appears to have a charge which changes with pH.
An intramolecular neutralization reaction leads to a salt-like ion called a zwitterion. The accepted practice is to show the amino acids in the zwitterion form.
- The carboxyl group can lose a hydrogen ion to become negatively charged.
- The amine group can accept a hydrogen ion to become positively charged.
5. Titration curve of Glycine
Glycine is optically inactive, simplest amino acid because it has no asymmetric carbon atom. Acid-Base titration involves the gradual addition (or) removal of protons. It has three different stages when the Glycine undergoes acid-base titration. Here is the complete material on the Titration curve of glycine.
6. Peptide Bond
“The peptide is formed between the amino group (-NH2) of the first amino acid and Carboxyl group (-COOH) of the second amino acid by eliminating one molecule of water.”
The amino acids are held together in a protein by covalent peptide bonds or linkages. These bonds are rather strong and serve as the cementing material between the individual amino acids. The peptide bond is a type of amide bond.
Salient features of Peptide bond:
- The peptide bond is rigid and planar
- The atoms in the peptide bond are Cα-C-N-Cα.
- The peptide bond is coplanar, this indicated a resonance or partial sharing of two pairs of electrons between the carbonyl oxygen and the amide nitrogen.
- The 4 atoms of the peptide group (C, H, O, and N) lie in a single plane, in such a way that the oxygen atom of the carbonyl group and the hydrogen atom of the amide nitrogen are trans to each other.
- The peptide bond shows a partial double bond character.
In this article, I have discussed the details of Amino acids. Read carefully and practice the structures, properties and observe the chemical change of amino acids. This is the major and basic chapter in all life science subjects. in the next article, I will post the details of major biomolecules like carbohydrates, Protein, Nucleic acids, and Lipids. Expecting to understand the concepts thoroughly.