Are you looking for the colour reactions of amino acids? BiochemistryDen shows the student some chemical reactions of proteins and amino acids with makeup proteins. These compounds are important parts of all living things, including the human body. The number of tests available shows how much time scientists have spent on this area of biological chemistry.
General Note on color reactions of amino acids
Some of the chemicals used in the following tests may be poisonous. They are safe to use if due care is taken in the laboratory.
Do not allow chemicals to enter the mouth, or to enter small cuts or scratches on the hands. Do not breathe in powders or allow them to blow around.
Always carefully wash your hands after an experiment and do what your teacher tells you to do to stay safe.
Color Reactions of Amino acids
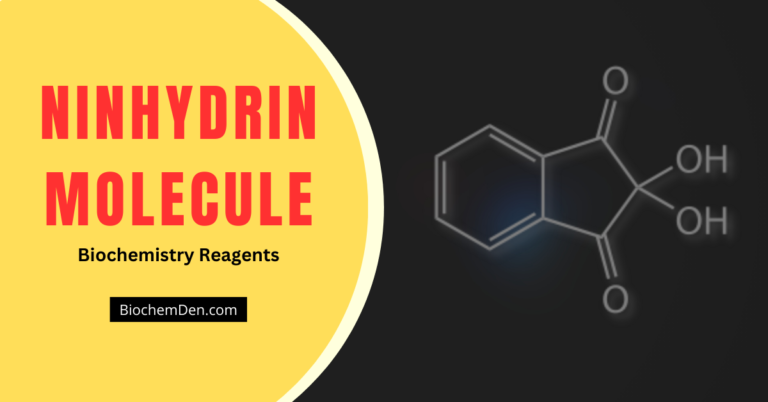
Experiment 1: Ninhydrin Test
Background
This test is widely used in biochemistry and food science. Although compounds other than proteins and amino acids also give positive reactions, standard procedures used in the analysis can make the reaction a positive test for amino acids and proteins.
Procedure
- Dissolve the contents of the vial marked “gelatin” in 100 mL water. In this case, more heat may be employed. Avoid boiling! Dissolve the contents of the vial marked “arginine” in 200 mL water.
- Do likewise with the contents of vials marked “glutamic,” “glycine,” “cysteine,” “tryptophan,” and “unknown.”
- Do not use the cysteine or tyrosine vials yet. Dissolve the contents of the vial marked “ninhydrin” in 200 mL ethanol.
- Set up a number of test tubes and into each, put 2.0 mL (34 drops) of ninhydrin reagent.
- Into each tube add 2 drops (0.1 mL) of each solution to be tested. Mix the solutions and boil in a water bath for 2 minutes.
- In this way, test albumin, gelatin, the unknown compound, and at least two of the ammo acids.
- If you wish to test cystine or tyrosine, simply add a speck of the powder to ninhydrin in one of the test tubes and boil with the others. (Note: The result and colors that develop)
Experiment 2: Biuret Test for Protein
Background
Biuret is a compound obtained when the area is heated to 1080C. It reacts with copper sulfate in an alkaline solution to give a violet color with an absorption maximum at 550 nm. This compound has given its name to the color reaction, which was also found to occur with other compounds-those having two or more amide groups or peptide bonds joined directly together, or through a single atom of carbon or nitrogen.
Procedure
- Use biuret reagent provided and each of the solutions prepared for Experiment 1.
- Set up a number of test tubes. Into each put 1.0 mL (17 drops) of biuret reagent.
- Then add the solution to be tested into the biuret tube (1.0 mL). Mix the solutions and note any changes in colour that occur over the next ten minutes.
- In this way, test albumin, gelatin, the unknown, and two amino acids.
Experiment 3: Solubility of Amino acids
You will already have dissolved a number of amino acids in water for the previous experiments. In this test, try to dissolve tyrosine and cysteine in water and note the result.
Procedure
- Put 2.0 mL (34 drops) of water in a clean test tube. Take a small portion of the contents of the vial marked “tyrosine” on a spatula and add it to the water.
- Shake well. Note if the tyrosine has dissolved. If it has not, then heat the test tube and note if the tyrosine dissolves.
- Is tyrosine readily soluble/sparingly soluble/insoluble in water? Repeat this procedure with cystine.
Experiment 4: Test for Cysteine and Cystine
The sulfur group of cysteine and cystine is liberated by heating with a strong alkali. If lead ions are present lead sulphide is formed as a dark precipitate. This reaction distinguishes between these two amino acids and methionine. Treatment with alkali does not liberate S from methionine.
Procedure
- Dissolve the contents of the vial marked “lead acetate” in 50 mL water. Dissolve the contents of the vial marked “NaOH” in 50 mL water. Put 20 mL aside for use in a later experiment.
- In 30 mL NaOH dissolves the cysteine, shaking well to ensure dispersal. Set up test tubes as follows: one with 2.0 mL glycine solution, one with 2.0 mL cysteine solution, one with 2.0 mL “unknown compound” and one with 2.0 mL cystine solution.
- To each tube add 2 drops of lead acetate solution, and heat the tubes for 5 minutes in a boiling water bath. Note the results.
Experiment 5: Sakaguchi’s Test for Arginine
Background
Ammonia and ammonium ions give positive reactions to this test. For this and other reasons, the reagents always give some colour in the reaction. Therefore, a blank or control containing reagents only should be prepared for comparison.
Procedure
- Prepare a 4% solution of NaOH by adding 20 mL 40% NaOH made up for experiment 4 to 180 mL water.
- Set up a number of test tubes as follows: one with 2.0 mL water, one with 2.0 mL arginine solution, one with 2.0 mL unknown compound, one with 2.0 mL glutamic acid solution, and one with 2.0 mL albumin solution.
- To each of these add 1.0 mL 4% NaOH solution, and then add 2 drops of alpha-naphthol reagent. Add 1.0 mL of sodium hypochlorite reagent to each solution and mix.
- Observe any colour change which occurs over the next 5 minutes. What colour is positive for arginine? Does albumin contain arginine?
Experiment 6: Xanthoproteic Test for Tyrosine and Tryptophan
Background
The reaction of concentrated nitric acid with some substituted aromatic rings gives a yellow colour (Xanthos = yellow in Greek).
Procedure
Set up test tubes as follows:
- In one tube put 2 drops of tryptophan solution, in another 2 drops of an unknown compound, in another 2 drops of glycine solution, and in another about 5 mg tyrosine powder.
- Then with great care, add 1.0 mL concentrated nitric acid (HNO3) to each test tube. Hold the tubes so that they do not point at you or anyone else.
- Heat the tubes gently in a water bath until they boil. Cool the tubes slowly and add the 4% NaOH, drop by drop, until the solutions are alkaline.
Did any colour change take place before the alkali was added? What was the final colour of each of the solutions?
Experiment 7: The Unknown Compound
Deduce the identity of the unknown compound from its various reactions.
Get this protocol in PDF format. Just download this “Color Reactions of Amino acids” file and make a print and distribute it to the students.
It helps you to protect your students from spelling mistakes and volumetric errors. All the best.