What is Tautomerization? Typically, this occurs as the migration of hydrogen atoms (protons) by an exchange of one single bond with a double bond.
In solutions where tautomerism is possible, a chemical equilibrium between the two tautomers is obtained.
The ratio of tautomers depends on many factors, including temperature, solvent, and pH. Tautomerism is a special case of structural isomerism.
Tautomerism may be catalyzed with acids and bases.
What is Tautomerization?
A tautomer is a separate type isomer by an organic compound that has the property that it can quickly change their isomeric form by a chemical reaction called tautomerization.
- Basic Components of Nucleic Acids – Purines and Pyrimidines
- Nucleic Acids: The Molecular Life Language Basics in Biology
The tautomers are couples of constitutional isomers inter-convertible by a reversible chemical reaction called tautomerization.
In most cases, the reaction occurs by the migration of Zinc atom of hydrogen accompanied by a change of location of a double bond.
In a solution of a compound capable of tautomerization, the equilibrium between the two tautomers is created. The ratio of tautomers is then depending on the solvent, temperature, and pH.
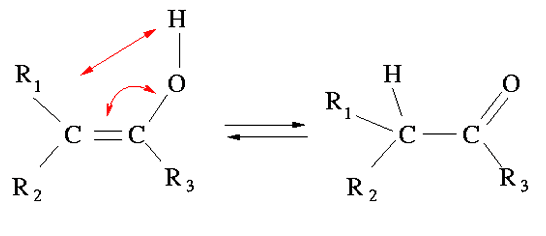
Tautomerism is the conversion of a functional group in another, most often by concomitant displacement of a hydrogen atom and a π bond (double or triple bond).
Unlike the mesomeric, the σ bonds (single bonds) may be displaced with the proton.
The prototypes are not tautomers, they do not induce a reorganization of the π system and hydrogen does not necessarily come from the same molecule (solvent).
Tautomerization is catalyzed by
Presence of bases
The mechanism is broken down into
- deprotonation,
- forming an anion delocalized,
- the protonation at a different place of the anion;
Presence of Acids
The mechanism is broken down into
- a protonation,
- forming a cation delocalized,
- the deprotonation at a different place of the cation.
Common tautomeric pairs in Tautomerization
- Keto-enol -tautomerism is the most common form of tautomerism and occurs in aldehydes, ketones and related compounds (essentially carbonyl compounds with one or more hydrogens on the α-carbon). Example: 2,4-pentanedione in equilibrium with the corresponding enol, in water are approximately 84% ketone and 16% as the enol.
- Phenol-keto tautomerism where a mold consisting of phenol and the other of the corresponding cyclic ketone. Example: 4-Pyrinol exists mainly in phenol form in the gas phase, but in its keto form in ethanol solution.
- Imine-enamine tautomerism is tautomerism where imines are in equilibrium with enamines. Generally, Imino the dominant form. Example: Isopropylidenemethylamine is usually in its imino form, but may, in certain reactions behave as an enamine.
- Ringtautomeri is tautomerism where hydrogen may sit in different positions on a ring. This tautomerism is often relatively slow. Example: 2,5-Dimethyl-1 H -imidazole may occur as 2,4-dimethyl-1 H -imidazole.
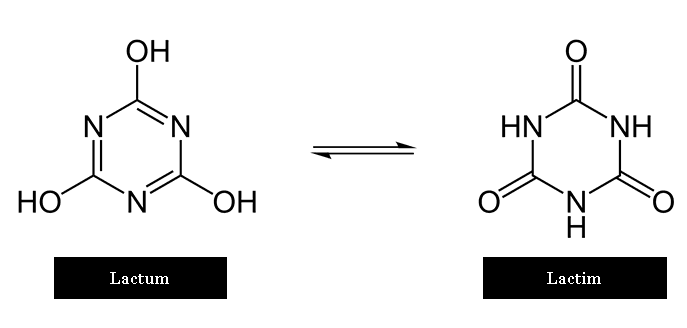
What is Tautomeric shift in Purine or Pyrimidine base?
The fact that the bases in DNA are not static was first pointed out by Watson and Crick.
Hydrogen atoms in the bases can move from one position in a purine or pyrimidine to another position.
Some of these are shown below. Such chemical fluctuations are Tautomeric shifts.
How tautomeric shift in a base in DNA may lead to mutation?
Tautomeric shifts causing mutations. The rare, less stable tautomeric forms of base exist for only very short periods of time.
However, if a base existed in the rare form at the moment that it was being replicated or being incorporated into a nascent DNA chain, a mutation might result: the rare imino or enol bases can form adenine-cytosine and Guanine-Thymine base pairs.
The net effect of such an event and the subsequent replication required to segregate the “mismatched” base-pair is an A-T to G-C or G-C to A-T base pair substitution.