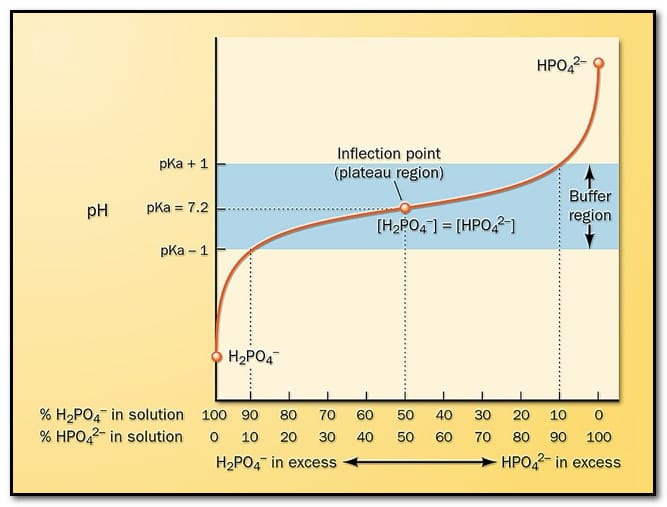
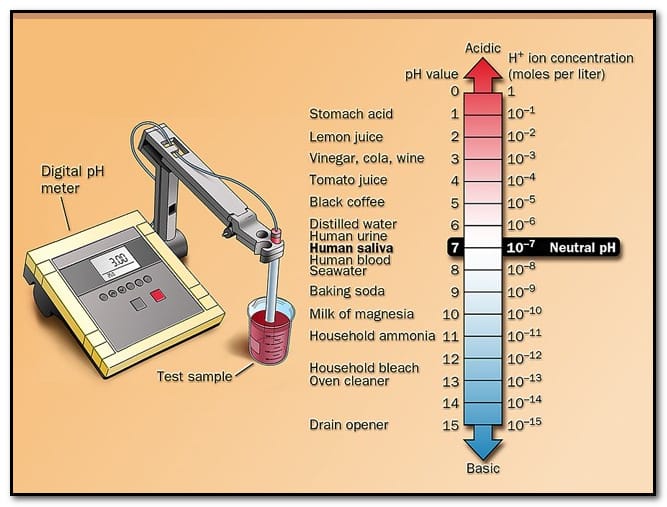
What are the basics of pH? The nature of the solution (acidic, alkaline (or) neutral) can be represented in terms of either hydrogen ion concentration or hydroxyl ion concentration.
In 1909, Sorenson used a logarithmic scale for expressing the H+ concentration. This scale was called “pH”, where “P” stands for “power” and “H” for hydrogen ion concentration.
Sorenson defines the pH of a solution as
“ The negative logarithm of hydrogen ion concentration (in moles/liter)”
Thus,
1
PH = log ————– = – log [H+]
[H+]
The symbol “P” denotes “negative logarithm of ”.
For a precisely neutral solution at 250C in which the concentration of hydrogen ions is 1.0 X 10-7 M, the pH can be calculated as follows:
1
PH = log———————= log (1.0 X 107)
1.0 X 10-7
=log 1.0 + log 107
= 0 + 7.0
= 7.0
The value of 7.0 for the pH of a precisely neutral solution is not an arbitrarily chosen figure. It is derived from the absolute value of the ion product of water at 250 C, which by convenient coincidence is a round number.
Importance of pH
- Soils of a specific pH are required for optimum group growth and better yields of crops.
- Specific pH values are to be maintained for the biological process and industrial process to occur.
- Specific pH is also to be maintained by the blood.
- PH plays an important role in chemical analysis.
Development reason for pH
- The pH scale was developed, taking water as the standard.
- It is an experimental fact that only 1 mole in 5,50,000,000 moles of water ionizes into an H+ and an OH-.
- This is the same proportion as one gram of hydrogen ion in 10,000,000 liters of water.
- Hence, one liter of water contains 1/10,000,000 (or) 1/107 of a gram of H+.
- For everyday use, only the ‘Power’ figure was used, and the symbol pH was placed before it.
Biological Significance of pH
A) Tautomeric forms of purines and Pyrimidines
Tautomerization is a special type of isomerism where a proton migrates in one direction and a covalent bond shifts in the opposite direction within the molecule. Purine and pyrimidine bases exist in different tautomerized forms according to pH. They are specific, tautomerized at the body PH of nearly 7.4 and are essential for the hydrogen bonding of complementary base pairs in the DNA double helices and RNA strands. So, pH maintains the natural three-dimensional forms of nucleic acid molecules.
B) Isoelectric pH
PH influences the ionization of ionizable polar groups of amino acids, proteins, nucleic acids, phospholipids, and mucopolysaccharides. At a specific pH, called the isoelectric pH of the molecule, each such molecule exists as dipolar zwitterions bearing both anionic acid and cationic groups and a minimum net charge. Zwitter ions do not migrate in electric fields and precipitate easily by aggregation due to minimum electrostatic repulsion.
C) Isolation of Proteins and Amino acids
The pH depends on the charged forms of proteins and amino acids are utilized in separating and isolating them from biological materials by methods such as “ion-exchange chromatography, Paper electrophoresis, and Isoelectrophoresis”.
D) Optimum pH
By influencing the ionized states of proteins, pH affects the ionic and hydrogen bonds that stabilize the three-dimensional structure of proteins.