ATP is synthesized by ATP Synthase, which is an enzyme complex made of a proton-conducting F0 unit and a catalyst F1 unit. The mitochondrial inner membrane contains the ATP synthesizing enzyme complex called ‘ATP synthase’(or) ‘F0 F1-ATPase’. (F for factor).
F1 component is like a “door-knob” protruding into the matrix from the inner membrane. It is attached by a stalk to F0 component, which is embedded in the inner membrane and extends across it, (“O” denotes, it is the protein of enzyme which binds the toxic antibiotic “Oligomycin”).
Thus the physiological role of the F1 component is to catalyze the synthesis of ATP.
The spherical F1 component (MW=360Kdal) contains a polypeptide chain subunits of five kinds (designated as α,β,γ,δ and ε) arranged into a cluster. It has many binding sites for ATP and ADP. The cuboid F0component is a hydrophobic segment of polypeptide chains.
F0 is the proton channel of the enzyme complex. The stalk is the communicating portion of the enzyme complex.
- What is Mitochondria in Biological Sciences
- Electron Transport Chain Components in Mitochondria
- Electron Transport Chain Mechanism in Mitochondria
- Oxidative Phosphorylation: Fate of Electrons in Mitochondria
Binding change mechanism of ATP Synthase:
The mechanism of ATP synthesis by proton – translocation ATP synthase can be conceptually broken down into three phases:
- Translocation of protons carried out by “F0”
- Catalysis of formation of the phosphor – anhydride bond of ATP carried out by F
- Coupling of the dissipation of the proton gradient with ATP synthesis, which requires interaction of F1 and F0.
The available evidence supports a mechanism for ATP formation proposed by “Paul Boyer”. According to this “binding change mechanism”, F1 has three interacting catalytic promoters, each in a different conformational state: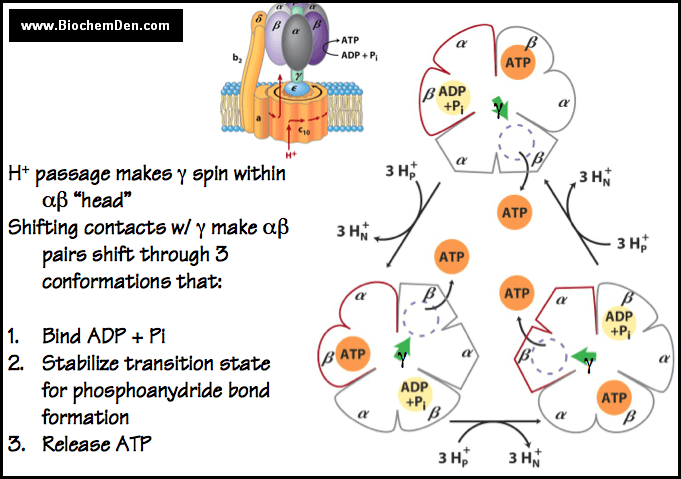
- L-State: That binds substrates and products loosely.
- T-State: That binds them tightly.
- O-State: That does not bind them at all (open state).
The free energy released on proton translocation is harnessed to inter-convert these three states. The phosphor anhydride bond of ATP is synthesized only in the “T-State”, and ATP is released only in the “O-State”. The reaction involves three steps.
- ADP and pi bond to the loose (L) binding site.
- A free energy driven conformational change converts the L-site to a tight (T) binding site that catalyzes the formation of ATP. This step also involves conformational changes of the other two promoters that convert the ATP-containing T-site to an open (o) site and convert the “O” site to an “L”-site.
- ATP is synthesized at the T-site on one subunit while ATP dissociates from the O-site on another subunit. The free energy supplied by the proton flow primarily facilitates the release of the newly synthesized ATP from the enzyme; that is, it drives the T-> O transition thereby disrupting the ‘enzyme-ATP’ interactions that had been promoted the spontaneous formation of ATP from ADP and pi in the T-site.
Source: Wikipedia