Ion exchange chromatography is a process that allows the separation of ions and polar molecules based on their affinity for the ion exchanger. It can be used for almost any kind of charged molecule, including large proteins, small nucleotides, and amino acids.
The ions are what move through the ion exchanger and can be detected by conductivity. This can be used to determine the concentration of an individual ion in a sample based on its conductivity. The conductivity can be used to quantify that ion and can be used with affinity chromatography.
W. Cohn first developed the ion exchange chromatography procedure. The reversible exchange of ions in solution with ions electrostatically bound to some sort of insoluble support medium. Ion exchange chromatography in protein purification has a very important role.

- Basics of Biochemical Techniques
- What is Paper Chromatography? Principle and Procedure
- Ninhydrin test or Ninhydrin reagent
What is ion exchange chromatography?
Ion exchange chromatography definition (or ion chromatography) is a process that allows the separation of ions and polar molecules based on their affinity for the ion exchanger. It can be used for almost any kind of charged molecule, including large proteins, small nucleotides, and amino acids.
The Ion exchange chromatography is a type of chromatography that uses an ion-exchange resin to remove ions from a solution. The resin consists of charged groups that bind to ions in the solution and remove them from the solution. Ion exchange chromatography is used to purify proteins and other biomolecules.
Principle of Ion-exchange Chromatography
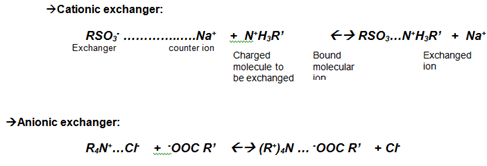
Ion exchange chromatography principle: the exchange of ions is the basic principle in this type of chromatography. In this process, two types of ion-exchange chromatography are used. They are, i.e., cationic and anionic exchangers can be used.
- Cationic exchangers possess negatively charged groups, and these will attract positively charged cations. These exchangers are also called “Acidic ion exchange” materials because their negative charges result from the ionization of acidic groups.

- Anionic exchangers have positively charged groups that will attract negatively charged anions. These are also called “Basic ion exchange” materials.
Types of Ion Exchange Resins
- Two main groups of materials are used to prepare ion exchange resins: Polystyrene and Cellulose.
- Resins made from these materials differ in their flow properties, ion accessibility and chemicals, and mechanical stability.
- Polystyrene resins are proposed by the polymerization reaction of styrene and divinylbenzene.
- A higher concentration of divinylbenzene produces higher cross-linkages.
- Polystyrene resins are very useful for separating small molecular weight compounds.
- Increasing the cross-linkage increases the rigidity, reduces swelling, reduces porosity & reduces the solubility of the polymeric structure.
- Sulfonic acids are strong acids with good proton dissociation ability. The sulfonation process makes acidic functional groups easily attached to nearly every aromatic nucleus.
- Resins substituted with sulfonic acid groups are cationic solid exchangers.
- Carbohydrate groups can be attached to the aromatic rings instead of a sulfonic acid group to prepare a weekly acidic exchanger.
- If primary functional groups are introduced, the resin can exchange anions rather than cations. Strong anion exchangers are prepared with a tertiary amine, yielding an entire quaternary ammonium group. Weak anionic exchangers can be prepared with secondary amines, yielding a weakly primary tertiary amine.
Cellulose resins have much greater permeability to macromolecular polyelectrolytes and possess a much lower charge density as compared to polystyrene exchangers.
- Carboxymethyl cellulose (CM-cellulose) – Cationic exchanger
- DEAE cellulose – Anionic exchanger
Preparation of the exchange medium
There are three steps are of absolute importance:
1) Swelling of medium: (Pre-cycling)
Swelling makes the functional groups to be exposed for ion exchange.
- The swelling of anion exchangers is usually carried out by treating it. first with an acid (0.5N HCl) and then with base (0.5N NaOH).
- Exactly the reverse is the case with cationic exchangers. The matrix can be treated with EDTA for impurity eliminations.
2) Removal of very small particles
These fines will decrease the flow rate and the unsatisfactory reaction. To remove fines, the exchanger is repeatedly suspended in a large volume of water and after the larger polymers have settled down, the slow sedimenting materials decanted.
3) Equilibration with counter ions
This is accomplished by washing the exchanger with different reagents depending upon the desired counter ion to be introduced.
- NaOH –> counter ion to be introduced is “Na+”
- HCl –> counter ion to be introduced is “H+”
- NaNO3 –> counter ion to be introduced is “NO3”
Choice of Buffers
- Anionic exchange Chromatography should be carried out with cationic buffers.
- Cationic exchange Chromatography should be carried out with anionic buffers.
- The pK of the buffer should be as near as possible to the pH at which the system is buffered. This results in high buffer capacity, which can easily withstand the local pH changes in the column.
| Buffers | PH range |
| Ammonium acetate | 4 to 6 |
| Ammonium formate | 3 to 5 |
| Pyridinium formate | 3 to 6 |
| Pyridinium acetate | 4 to 6 |
| Ammonium carbonate | 8 to 10 |
Ion-Exchange Chromatography Procedure
- Ion exchange separations are carried out mainly in columns packed with an ion-exchanger. These ionic exchangers are commercially available, and they are made up of styrene and divinylbenzene.
- DEAE-cellulose is an anionic exchanger, and CM-cellulose is a cationic exchanger. The choice of the exchanger depends upon the particle’s charge to be separated. To separate anions, “Anionic exchanger” is used. For different cations, “Cationic exchanger” is used.
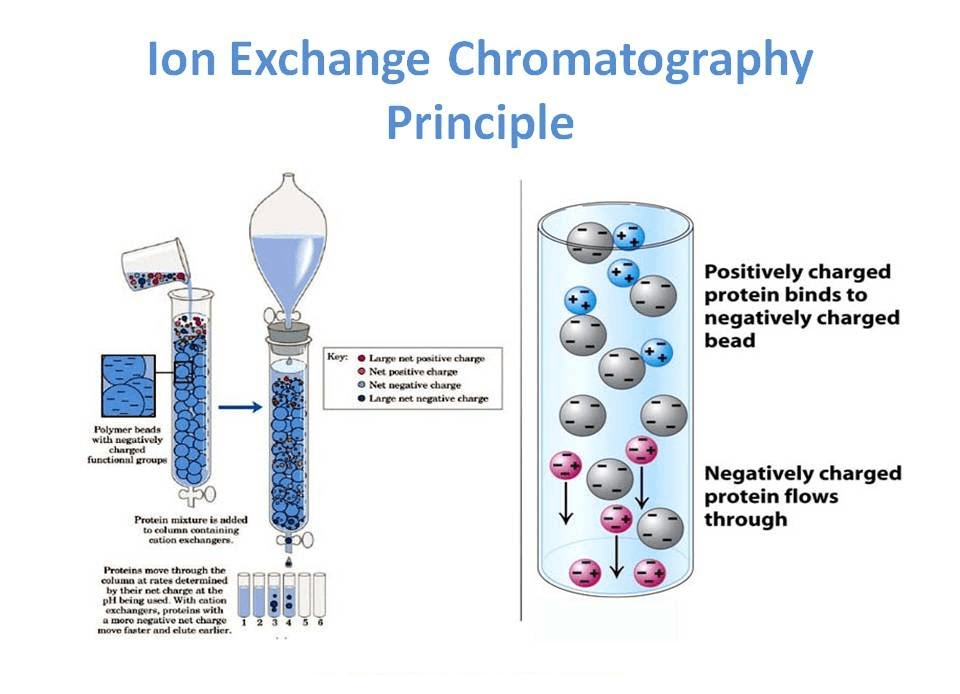
- First, the ion exchange chromatography column is filled with an ion exchanger, then the sample is applied, followed by the buffer. Tris, pyridine, acetate, citrate, and phosphate buffers are widely used.
- The particles that have a high affinity for ion exchangers will come down the column along with buffers. In the next step, the corresponding buffer separates the tightly bound particles.
- Then these particles are analyzed spectroscopically.
Ion exchange chromatography applications
- It is highly used in the analysis of amino acids. The amino acid “Autoanalyzer” is based on in exchange principle.
- To determine the base composition of nucleic acids. Chargaff used this technique to establish Adenine and Thymine’s equivalence; Guanine and Cytosine.
- This is the most effective method for water purification. Complete deionization of water (or) a non-electrolyte solution is performed by exchanging solute cations for hydrogen ions and solute anions for hydroxyl ions. This is usually achieved by the method used to soften drinking water.
- Protein purification by ion-exchange chromatography is another successful technique.
- It is also used to separate many vitamins, other biological amines, and organic acids and bases.
One of the main disadvantages of ion-exchange chromatography is its buffer requirement. Because binding to IEX resins is dependent on electrostatic interactions between proteins of interest and the stationary phase, the ion-exchange chromatography column must be loaded in low-salt buffers. The ion-exchange chromatography for protein purification is the most popular method in biochemical techniques.
This restriction may require a buffer exchange step before ion-exchange chromatography for some applications.
Final words
If you’re looking for an easy way to separate components from a mixture, ion exchange chromatography is excellent. One of the most significant advantages of this type of chromatography is that the process is relatively fast. Another advantage is that it doesn’t require a lot of equipment, and the process can be scaled up depending on the size of the sample.