Amino acids are the basic building blocks of proteins. This chapter includes the physiological properties of amino acids. Before going to the physio chemical properties of amino acids, we should brush back on the amino acid basic points.
What is an amino acid? Amino acids are biological organic molecules containing both an amino group (s) and a carboxyl group (s).

An amino acid is an organic molecule with an amino group (NH2) and a carboxyl group (COOH). The most frequent and of the greatest interest are those amino acids that form part of proteins.
Two amino acids are combined in a condensation reaction between the amino group and the carboxyl group of another amino acid, releasing one molecule of water and forming an amide bond. This is called a peptide bond.
These two amino acids form a dipeptide. If you add a third amino acid, one tripeptide is formed, and so on, to create a polypeptide. This reaction occurs naturally within cells, in ribosomes.
All components of the protein amino acids are L-alpha-amino acids. This means that the amino group is attached to the carbon adjacent to the carboxyl group (alpha carbon), or, in other words, both the carboxyl and the amino are attached to the same carbon; this one-unit alpha carbon-hydrogen and a chain (usually called a side chain or radical R) of the variable structure determine the identity and properties of each of the different amino acids.
Hundreds of radicals so hundreds of different amino acids are known, but only 22 (the latter two were discovered in 1986 – selenocysteine - and 2002, – pyrrolysine -) 2 are part of proteins and have codons at specific genetic code.
The human body is made up of 20 percent protein. Proteins play a key role in almost all biological processes. Amino acids are the basis of proteins.
Since many of our cells, muscles, and tissues are composed of amino acids, they perform many important functions in our body: they give the cell not only its structure but are also responsible for transporting and storing all kinds of vital nutrients. Amino acids influence the functions of organs, glands, tendons, and arteries.
They are essential for wound healing and tissue repair, especially muscles, bones, skin, and hair, as well as eliminating the negative impacts associated with metabolic disorders of all types.
This topic included the complete list of the physiochemical properties of amino acids.
Physical and Chemical Properties of Amino acids
Let us discuss the physical and chemical properties of amino acids. Here are the physical properties of amino acids.
1. Solubility
Most of the amino acids are usually soluble in water and insoluble in organic solvents.
2. Melting Point
Amino acids are generally melted at a higher temperature of ten above 2000C.
3. Taste
Amino acids may be sweet (Gly, Ala & Val), tasteless (Leu) or Bitter (Arg & Ile).
4. Optical Properties
All amino acids possess optical isomers due to the presence of asymmetric α-carbon atoms.
5. Zwitter ion and Isoelectric point
The name “Zwitter” is derived from a German word that means “hybrid”. Zwitter ion (or) (dipolar ion) is a hybrid molecule containing positive and negatively ionic groups.
Basically, the proton shifts from the carboxyl group to the amino group of the self molecule at normal pH cellular levels.
6. Titration Curve of Glycine
Glycine is optically inactive and the simplest amino acid because it has no asymmetric carbon atom. Acid-base titration involves the gradual addition (or) removal of protons.
It has three different stages when the glycine undergoes acid-base titration.
Chemical Properties of Amino acids
Here are the chemical reactions of amino acids due to carboxyl and amino groups
I) Due to the Carboxyl group
a) Decarboxylation
The amino acids will undergo alpha decarboxylation to form the corresponding “amines.” Thus, important amines are produced from amino acids.
- Histidine Histamine + CO2
- Tyrosine –Tyramine + CO2
- Tryptophan Tryptamine + CO2
- Lysine Cadaverine + CO2
- Glutamic acid Gamma Amino Butyric Acid (GABA) + CO2
b) Reaction with Alkalies (Salt formation)
The carboxyl group of amino acids can release an H+ ion with the formation of carboxylate (COO–) ions. These may be neutralized by cations like Na+ and Ca+2 to form salts. Thus, amino acids react with alkalies to form “salts.”
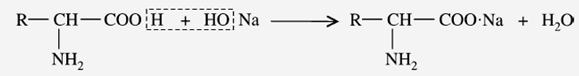
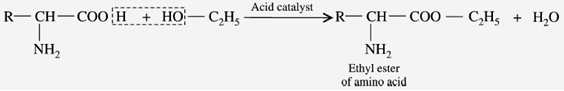
c) Reaction with Alcohols (Esterification)
When the amino acids are reacted with alcohol to form “ester”. The esters are volatile, in contrast to the form of amino acids.
d) Reaction with Amines
An amino acid reacts with amines to form “amides”.

II) Due to Amino group
a) Reaction with Mineral acids (Salt formation)
When the amino acids are treated with mineral acids (like HCl), they form “acid salts”.
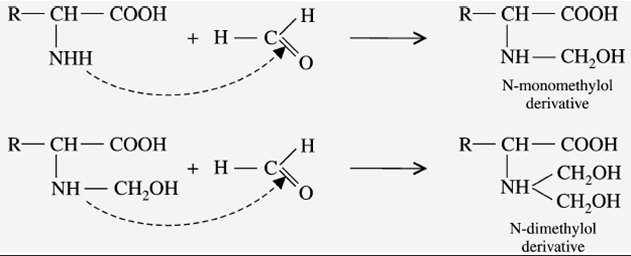
b) Reaction with Formaldehyde
When the amino acid reacts with two molecules of formaldehyde, it forms an “N-dimethylol derivative” (hydroxy-methyl derivative). This reaction is done in two steps. These derivatives are insoluble in water and resistant to attack by microorganisms.
c) Reaction with Benzaldehyde
When the amino acid reacts with Benzaldehyde, it gives “Schiff’s base”.

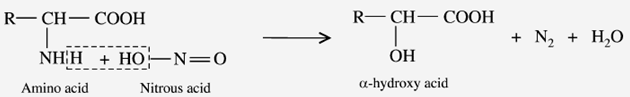
d) Reaction with Nitrous acid (Van Slyke reaction)
When the amino acids react with nitrous acid (HNO2) to liberate N2 gas and produce the corresponding “-hydroxyl acid”. The imino acids proline and hydroxyproline do not respond to this reaction.
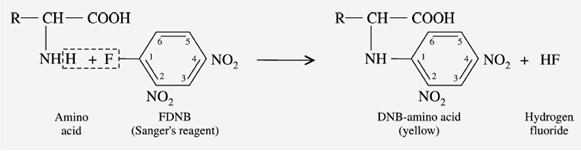
e) Reaction with Sanger’s reagent
“1-fluoro-2,4-dinitrobenzene” is called Sanger’s reagent (FDNB). In a mildly alkaline solution, the Sanger’s reagent reacts with -amino acid to produce a yellow-colored derivative, DNB-amino acid.
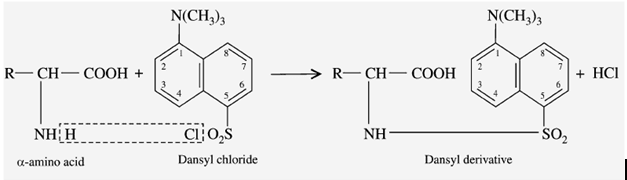
f) Reaction with DANSYl Chloride
DANSYL chloride means “Dimethyl Amino Naphtha Sulphonyl Chloride”. When the amino acid reacts with the DANSYL chloride reagent, it gives a “fluorescent DANSYl derivative”.
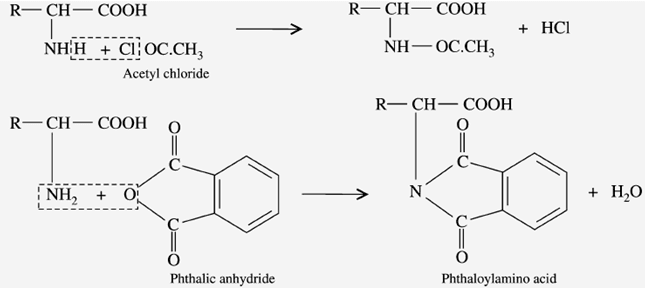
g) Reaction with acylating agents (Acylation)
When the amino acids react with “acid chloride” and acid anhydride (phenic anhydride) in an alkaline medium, it gives “pthaloyl amino acid”.
c) Due to amino & carboxyl group
Ninhydrin reaction
Step1:
Ninhydrin (=indane 1,2,3-trione hydrate) is a powerful oxidizing agent and causes oxidative decarboxylation of α-amino acids, producing CO2, NH3, and an aldehyde with one fewer carbon atom than the parent amino acid.
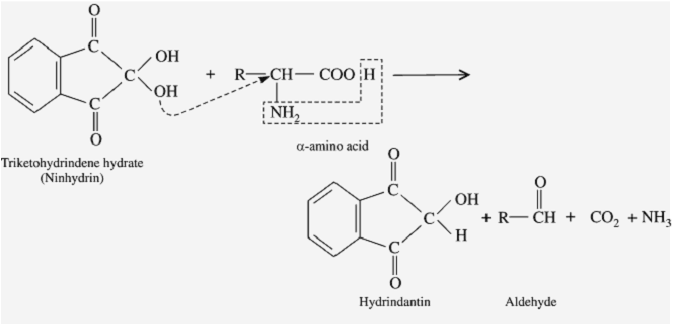
Step2:
The reduced ninhydrin then reacts with the liberated NH3 and a mole of ninhydrin, forming a blue-colored Rhumann’s complex.
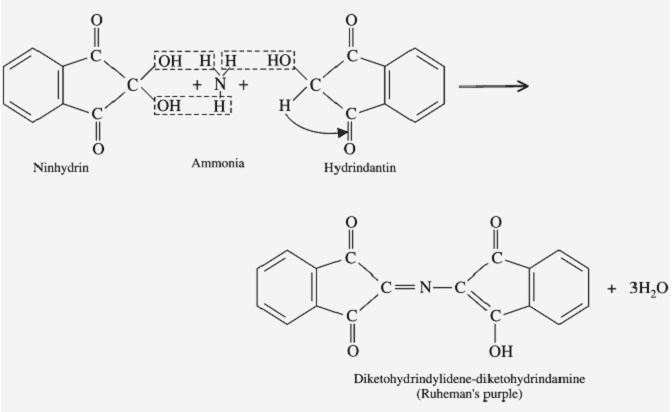
This reaction is a very sensitive reaction and it is used for amino acid and imino acid identification.
When Amino acids (or) Imino acid reacts with the Ninhydrin molecule it gives Color. When it gives Purple color (Rhumann’s Complex) –the Unknown sample is Amino acids (Which have primary amine –NH2) or it gives Yellow color – the Unknown sample is Imino acid (-NH-).
Reaction with Edmann’s degradation
Erdmann’s reagent is “phenylisothiocyanate.” When amino acids react with Edmann’s reagent, it gives “phenyl thiohydantoic acid.” Finally, it turns into the cyclized form “Phenyl thiohydantoin” (Edmann’s derivative).
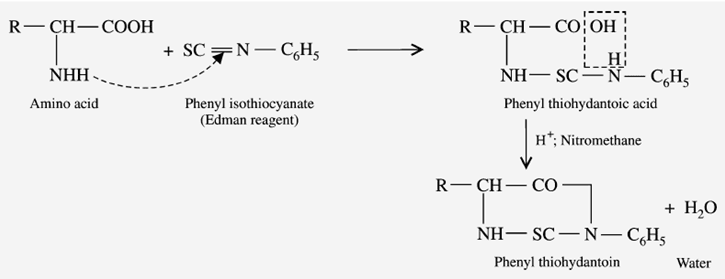
The physical and chemical properties of amino acids give positive results after completing the reaction.
Reference readings: Prediction of Physical-Chemical Properties