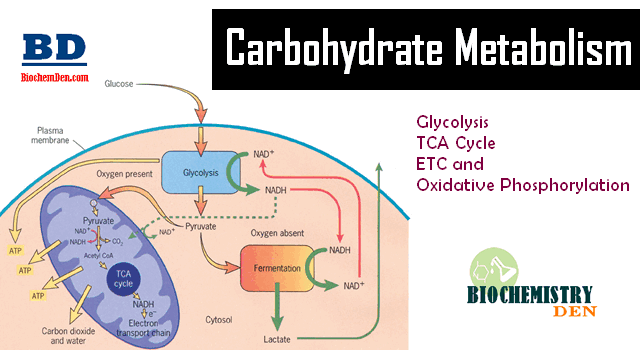
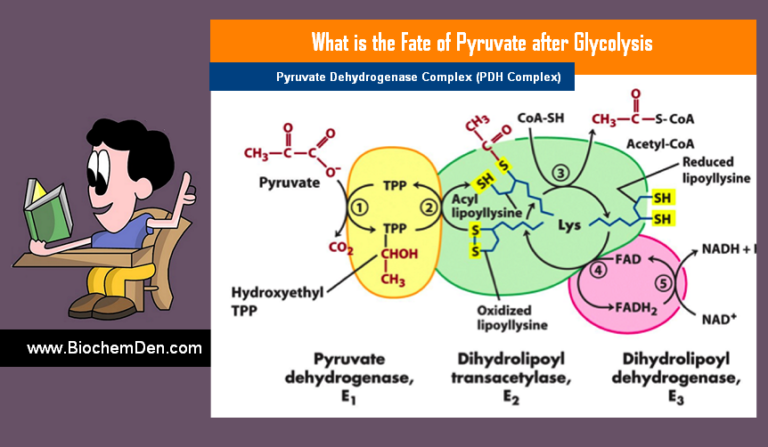
Gluconeogenesis is the process of synthesizing glucose from non-carbohydrate sources. Where does gluconeogenesis occur? The process takes place mainly in the liver and to a limited extent in the kidney and small intestine under some conditions.
It is also called “endogenous glucose production” (EGP). It is one of the metabolic pathways.
The production of glucose from other carbon skeletons is necessary since the testes, erythrocytes, and kidney medulla exclusively utilize glucose for ATP production.

Why is gluconeogenesis important? During neoglucogenesis, the glycogen (the storage form of animal starch) is made with long chains of glucose molecules. It is broken down into glucose, which then enters the blood.
Gluconeogenesis definition
The definition of Gluconeogenesis is given below
The biosynthesis of carbohydrates from simpler, non-carbohydrate precursors such as oxaloacetate and pyruvate is called “gluconeogenesis“.
(or)
“Synthesis of Glucose / Carbohydrates from Non-carbohydrate precursor molecules.”
- The starting point precursor of the pathways is a pyruvic acid molecule, although oxyaloacetic acid and dihydroxyacetone phosphate also provide entry points.
- Lactic acid comes from some amino acid metabolism and glycerol (from fat).
- It is similar but not the exact reverse of glycolysis, some steps are identical in the reverse direction, and three of them are new ones.
- Glycolysis and gluconeogenesis are both reversible processes.
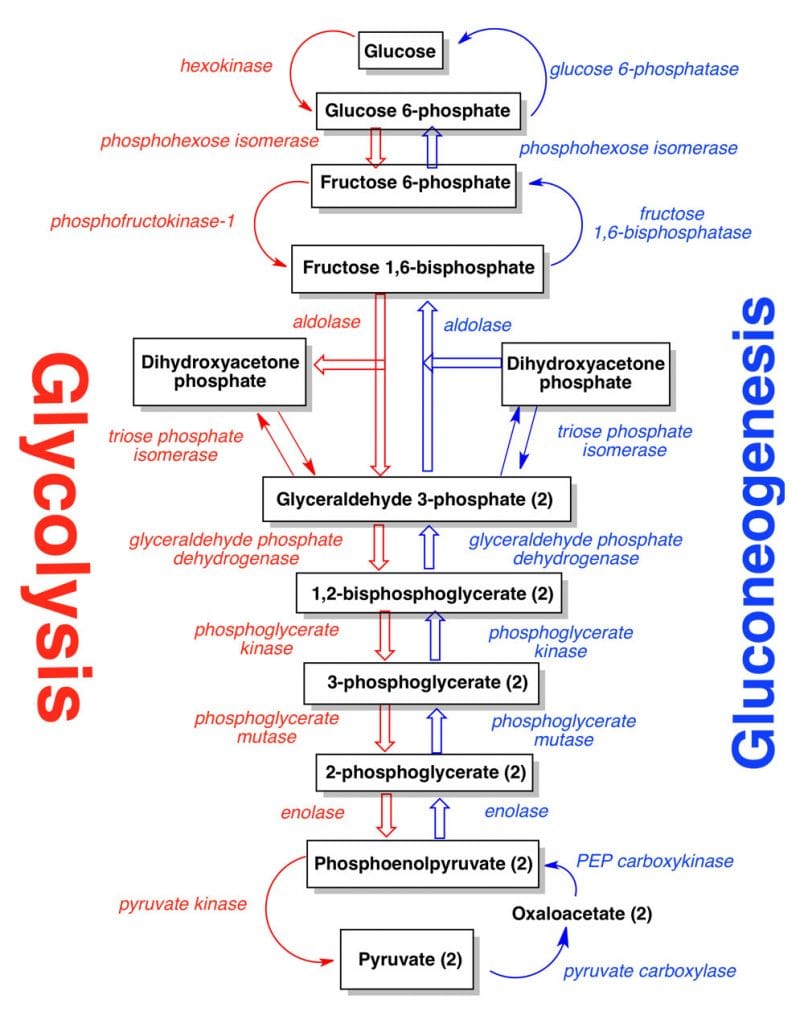
- Without going into detail, the general neoglucogenesis sequence is given in the graphic on the left.
- Notice that oxaloacetic acid is synthesized from pyruvic acid in the first step. Oxaloacetic acid is also the first compound to react with acetyl CoA in the citric acid cycle. The concentration of acetyl CoA and ATP determines the fate of oxaloacetic acid.
- If the concentration of acetyl CoA is low and the concentration of ATP is high, then neoglucogenesis proceeds. Also, notice that ATP is required for the biosynthesis sequence of the pathway.
- Gluconeogenesis occurs mainly in the liver, with a small amount also occurring in the cortex of the kidney. It occurs very little in the brain, skeletal muscles, heart muscles, or other body tissue. In fact, these organs have a high demand for glucose.
- Therefore, this pathway is constantly occurring in the liver to maintain the glucose level in the blood to meet these demands.
Steps in Gluconeogenesis
Synthesis of glucose from pyruvate utilizes many of the same enzymes as Glycolysis. Here is the gluconeogenesis pathway.
Kreb’s pointed out that energy barriers obstruct a simple reversal of glycolysis.
- Between Pyruvate and PEP (Enzymes: Pyruvate Carboxylase and Phosphoenolpyruvate Carboxylase-PEPCK)
- Between Fructose-1,6-bis P and Fructose-6-P (Enzymes: Fructose-1,6-bisphosphate)
- Between Glucose-6-P and Glucose (Enzymes: Glucose-6-Phosphatase)
- Between Glucose-1-P and Glycogen (Enzyme: Glycogen Synthase)
Glycolysis has three reactions that have a forward direction in that they are essentially irreversible (see lecture notes on Glycolysis):
- Hexokinase (or Glucokinase),
- Phosphofructokinase, and
- Pyruvate Kinase.
These steps must be bypassed in gluconeogenesis. Two of the bypass reactions involve simple hydrolysis reactions.
Below is the forward reaction catalyzed by each of these Glycolysis enzymes, followed by the bypass reaction catalyzed by the Gluconeogenesis enzyme.
Step 1: Glucose Phosphorylation/Dephosphorylation
In Glycolysis, the first step is phosphorylation.
Glucose + ATP –> Glucose-6-phosphate + ADP
Enzyme: Hexokinase or Glucokinase (Glycolysis)
In gluconeogenesis, the first step in glycolysis is reversible.
Glucose-6-phosphate + H2O –> glucose + Pi
Enzyme: Glucose-6-phosphatase
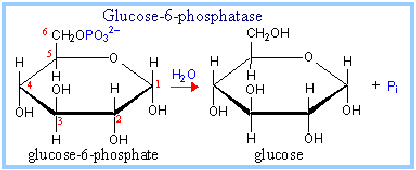
The glucose-6-phosphatase enzyme is embedded in the endoplasmic reticulum (ER) membrane in the liver of the cells.
Evidence indicates that the catalytic site is exposed to the ER lumen. Another subunit of the enzyme is postulated to function as a translocase, providing substrate access to the active site.
Step 2: Fructose Phosphorylation/Dephosphorylation
In Glycolysis, Step 3 is
Fructose-6-phosphate + ATP –> fructose-1,6-bisphosphate + ADP
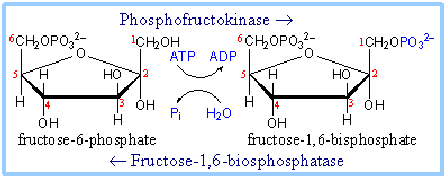
Enzymes: Phosphofructokinase
In gluconeogenesis, step 3 in glycolysis is reversible.
Fructose-1,6-bisphosphate + H2O –> fructose-6-phosphate + Pi
Enzyme: Fructose-1,6-Bisphosphatase
Step 3: Pyruvate Phosphorylation/Dephosphorylation
In glycolysis, step 9 is
Phosphoenolpyruvate + ADP –> pyruvate + ATP
Enzyme: Pyruvate Kinase
For bypass of the Pyruvate Kinase reaction of glycolysis, cleavage of 2 ~P bonds is required. The free energy change associated with the cleavage of one P bond of ATP is insufficient to drive the synthesis of phosphoenolpyruvate (PEP) since PEP has a higher negative DG of phosphate hydrolysis than ATP.
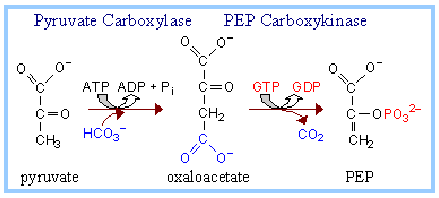
In Gluconeogenesis,
The two enzymes that catalyze the reactions for the bypass of the Pyruvate Kinase reaction are the following:
a) PEP Carboxylase Reaction
Pyruvate + HCO3– + ATP –> Oxaloacetate + ADP + Pi
Enzyme: Pyruvate Carboxylase
(b) PEP Carboxykinase Reaction:
Oxaloacetate + GTP –> Phosphoenolpyruvate + GDP + CO2
Enzyme: PEP Carboxykinase
Contributing to the spontaneity of the two-step pathway are the following:
- The free energy of cleavage of one ~P bond of ATP is conserved in the carboxylation reaction. Spontaneous decarboxylation contributes to the spontaneity of the second reaction (PEP synthesis).
- Cleavage of a second ~P bond of GTP also contributes to driving the synthesis of PEP.
About Biotin Vitamin
Pyruvate Carboxylase utilizes biotin as a prosthetic group.
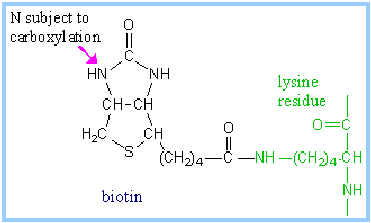
- Biotin has a 5-carbon side-chain whose terminal carboxyl is in an amide linkage to the e-amino group of lysine of the enzyme.
- The biotin and lysine side chains together form a long swinging arm that allows the functional group of biotin to swing back and forth between two active sites.
- Biotin carboxylation is catalyzed at one active site by pyruvate carboxylase.
- ATP reacts with HCO3– to yield carboxy phosphate. The carboxyl is transferred from this ~P intermediate to N of a ureido group of the biotin ring system.
Overall:
Biotin + ATP + HCO3– –> carboxy-biotin + ADP + Pi
Biotin-dependent enzymes in animals
| Enzyme | Role |
| Pyruvate Carboxylase | The first reaction in a pathway that converts 3-carbon precursors to glucose (gluconeogenesis) |
| Acetyl~coA carboxylase | Commits acetate units to fatty acid synthesis by forming malonyl~coA |
| Propionyl~coA Carboxylase | Converts propionate to succinate, which can then enter the citric acid cycle. |
| beta-Methylcrotonyl~coA carboxylase | Catabolism of leucine and certain isoprenoid compounds |
Other Reactions in Gluconeogenesis
1. Pyruvate Carboxylase
The enzyme converts pyruvate to oxaloacetate, which is allosterically activated by acetyl coenzyme A. The adaptive value of this regulation relates to the interconnectedness of the pathways, as shown at right.

Acetyl CoA enters the Krebs Cycle by condensing with oxaloacetate, whose concentration tends to be limiting for the Krebs Cycle. When gluconeogenesis is active in the liver, oxaloacetate is diverted to form glucose (via PEP).
Oxaloacetate depletion hinders acetyl CoA entry into the Krebs Cycle. The resulting increase in [acetyl CoA] activates pyruvate carboxylase to synthesize more oxaloacetate.
2. Lactate to Glucose
The major breakdown product of anaerobic glycolysis in muscle is lactic acid. In muscle tissue, it’s called lactic acid. Muscle tissue is, however, not capable of re-synthesizing glycogen from lactate.
This conversion, therefore, takes place entirely in the liver. Muscle lactate is transported by the blood to the liver, where it is converted into glucose and glycogen by enzymes involved in gluconeogenesis.
Liver glycogen then breaks down into glucose and is carried back to the muscles by the blood. This conversion of muscle lactic acid to glucose in the liver and its re-entry into muscle is called the Cori cycle.
3. Amino acids to Glucose
The major portion of glucose formed in gluconeogenesis comes from amino acids. Glycogenic amino acids are converted into either citric acid cycle intermediates or pyruvate. These substances come from glucose in the liver.
Pyruvate is carboxylated to form OAA by pyruvate carboxylase and ATP in mitochondria. Further reactions in the formation of glucose take place in the cytoplasm, and therefore, oxaloacetate must come out of the mitochondria.
Oxaloacetate, however, does not readily permeate through the mitochondrial membrane and thus requires conversion to a compound that could diffuse out of the mitochondria. This is achieved mainly by its conversion to malate, which readily passes through the mitochondrial membrane. In the cytoplasm, malate is reconverted to oxaloacetate.
Oxaloacetate is then decarboxylated to form phosphoenolpyruvate (PEP) by phosphoenolpyruvate carboxylase and GTP. The conversion of phosphoenolpyruvate (PEP) to fructose-1, 6-diphosphate is carried out by enzymes of glycolysis found in all tissues.
The hydrolysis of fructose diphosphate to form fructose-6-phosphate requires a specific fructose diphosphatase. Fructose-6-phosphate also requires a specific glucose-6-phosphatase for its conversion to Glucose. Both the abovementioned specific enzymes are found only in liver and kidney tissues.
4. Glycerol to Glucose
Glycerol arising from the breakdown of triacylglycerides is also a good source for the synthesis of glucose in the liver. It requires initial phosphorylation by ATP followed by reduction to form DHAP (Dihydroxyacetone phosphate), which enters the pathway of gluconeogenesis.
Importance of Gluconeogenesis
- A continual supply of glucose is necessary as a source of energy, especially for the nervous system and the erythrocytes.
- A gluconeogenesis mechanism is used to clear the products of the metabolism of other tissues from the blood, e.g., lactate, produced by muscle and erythrocytes, and glycerol, which is continuously produced by adipose tissue.
How is Glycogenolysis regulated?
- Glucagon and glucocorticoids increase NeoGlucogenesis.
- Insulin inhibits the process.
- When glucagon levels rise, the cellular cAMP levels also increase. The cAMP concentration inactivates the enzyme Pyruvate dehydrogenase (PDH) by a phosphorylation process. This would ensure that more pyruvate is converted to oxaloacetate and thereby channel neoglucogenesis.
- Ethanol inhibits this pathway.
- Glyconeogenesis and glycolysis have opposite directions; their reactions to regulatory signals may be opposite, or they work against one of the Futile cycles.
The summery has been given in the below table
| Enzyme | Activation | Inhibition |
| Pyruvate Carboxylase | Cortisol, Glucagon, Adrenalin, Acetyl CoA | Insulin, ADP |
| PEPCK | -do- | Insulin |
| Fru-1,6-bis-Phosphate | -do- | Fru-1,6-Bis P, AMP, Fru-2,6-BP |
| Glc-6-Phosphatase | -do- | Insulin |
What is gluconeogenesis, and where does it occur?
Gluconeogenesis, also called glycolysis from glucose and keto-acidosis from beta-hydroxybutyrate, – occurs during prolonged starvation when there is no dietary or endogenous carbohydrate (a process called fasting gluconeogenesis). Glucose plus the enzyme hexokinase is converted into glucose 6P, which later in the cycle goes to 60K.
If a mammal lacks insulin; it cannot use fat as food but can still break down glycogen and ketogenic amino acids in the liver, so they produce glucose 6P by a process called gluconeogenesis. Prolonged fasting usually leads to nutritional ketosis (some sources say “fat loss,” but this is just not possible during starvation). Because proteins don’t get used up normally, it’s going to take more than calories from protein alone to maintain the maintenance needs.
The need to use glucose is paramount, so a large body directly converts fat and ketogenic amino acids into glucose 6P using gluconeogenesis, or glycolysis (which happens in the liver during prolonged fasts) thanks to the hexokinase enzyme. Since it is called “glucose coming out,” it has nothing to do with glucose coming out of the cells—it’s about generating more glucose for fuel during starvation periods.
If a mammal without insulin cannot produce anything other than pyruvate (also known as dihydroxyacetone phosphate) from fat breakdown; its body is headed towards gluconeogenesis and ketoacidosis.
Not only that, but several studies have shown conflicting results on the timing of keto-adaption, suggesting that glucose is produced while fasting and the extra glucose then exerts a “thesis” on the cells, causing keto-adaptation, in which case it would sometimes be termed “Glucocorticoid-induced Keto-Adaption.”
This study suggests that gluconeogenesis takes place during starvation as well, but on longer timescales compared to periods of just eating.
How do you know if gluconeogenesis is working?
The brain is the most metabolically active part of your body, and glucose (sugar) will be converted back into muscle energy when it’s not being used.
When there are shortages of sugar, known as hypoglycemia, this occurs in a higher than usual quantity for a few hours to days before dropping as normal glucose levels return—this can leave people groggy, dizzy, or lethargic.
The sort of hypoglycemia you experience is determined by the amount of sugar in your food and how quickly glucose is broken down via gluconeogenesis to keep up with metabolism.
Glucose has a half-life of only two hours in humans when normal levels are maintained; therefore, it will become dangerously low in glucose for brain function.
Many people struggling with excess body fat may manage this by eating less, mainly as we can’t store more than a certain amount of stored energy in our adipose tissue, which is an accumulation of the fat that accumulates from food. It might be wise to reduce your calories instead, rather than stocking up on extra energy to overcompensate and give you weight control problems down the line.
Frequently Asked Questions (FAQs) on Gluconeogenesis
What is Gluconeogenesis?
Gluconeogenesis is the first 18 steps in our breakdown of stored carbohydrates (glucose) into glucose. In actuality, these are like 19 steps because we eliminate 1 input step along the way; it’s why some medical professionals think that a person who dies from starvation still has medium-chain triglycerides left over due to one extra metabolism product removing itself or performing its own removal earlier than elsewhere in his body.
What are glycogenolysis and gluconeogenesis?
These processes occur together and with glycolysis but to different ends. Glycogenolysis is a process by which glycogens are broken down into glucose molecules during our starvation period that follows eating carbohydrates rather than fat or protein (or ketones). Steps involved include phosphorylation, oxidation, and splitting up at the β-glycosidic bond to enter glycerol 3-phosphate dehydrogenase, which makes them access the Krebs cycle for acetyl-CoA, a metabolite that enters mitochondria. Those converted from glucose are transported into glycolysis (the first and most prominent three metabolic pathways). Pyruvate is oxidized by substrate-level phosphorylation to carbon dioxide during protein breakdown through citric acid or aconitase as well as oxaloacetate via Coenzyme Q. A esters thanks to AMP-activated protein kinases and pyruvate carboxylase are then oxidized to lactic acid. The glucose will continue and be dispersed throughout the cell via gluconeogenesis (if not from glycogenolysis). Unneeded glucose can also be utilized as an energy source for many other metabolic processes within your body by using it through glycolysis. For example, it can be broken down into ATP during the breakdown of fatty acids and amino acids or indirectly into other metabolites such as pyruvate.
Why is it important to have carbohydrates if you’re trying not to gain weight?
If a diet includes too few carbohydrate calories for your body’s needs (e.g., if one follows a severely low-carbohydrate regimen), glycogen stores are depleted; thus, glycogenesis will continue for blood glucose levels to be maintained. If that does not happen, we will store fat—it just helps us survive the starvation period by creating new cells and increasing our metabolic efficiency during periods when no food is available (e.g., the near-death experience).
Why did it take so long for mitochondria to evolve?
Carbon dioxide didn’t exist in prebiotic chemistry; there were other things, like acetate/format, which could use for carbon dioxide in the process of fermentation. Also, mitochondria seem to have lost their function during evolution from cell anaerobes to gram-negative bacteria (with complex organic molecules required for ATP generation). Mitochondrial DNA was recently sequenced by sequencing a few genes, and about 90% of them were identical. “Mitochondrial DNA is maternally inherited.” Another essential point is that aerobic reactions are thermodynamically far more favorable. Also, remember that hypoxia generally occurs in tissues and cells where anaerobic metabolism can generate energy for prolonged periods (e.g., our muscles during exercise).
Why does ATP activate gluconeogenesis?
Basically, like ketogenesis, gluconeogenesis is just a way for the liver (during starvation) to maintain its namesake. It does this with glucose being broken down into pyruvic acid through glycolysis and further used by several metagenic pathways that flood your body’s cells with ATP. Some of these steps involve stepwise addition of AMP or ADP along the way, but many others do not; i.e., the steps listed above do not. For the act of gluconeogenesis to proceed, these 11 additional metabolic pathways must first be activated and not inhibited or deleted way down the line (pretty much).
How does glucagon activate glycogenolysis?
In a normal state of protein and nucleotide catabolism, amino acids are originally given carbon skeletons by the combination of glycolysis and the citric acid cycle (glucose is broken down into pyruvic acid), known as oxidative metabolism.
There are even more alternate metagenic pathways that use AMP’s intermediate product ATP to form other molecules than just acetyl-CoA, which could be used in gluconeogenesis. If glucose levels drop low enough, the liver will just start using amino acids directly to generate ATP, which can be used locally in a sort of anaerobic metabolic loop.
Clinical Significance
- In the enzyme pyruvate carboxylase, a deficiency is seen as an inborn error of metabolism, where mental retardation is manifested.
- Its incidence is one in every 25,000 births.
- The pyruvate carboxylase gene is located on human chromosome No. 11.
- In type II diabetes mellitus conditions, the rise in gluconeogenesis is responsible for the production of excess glucose after an overnight fast.